This procedure is performed to relieve pain or muscle spasm in patients whose pain has not been controlled with more conservative therapies. The benefits for intrathecal pump placement include a reduction in side effects such as nausea, vomiting, sedation and constipation. Other benefits include decreased need for oral medications and increased ability to perform activities of daily living. An implantable system may reduce the risk of infection compared to the long-term use of external systems. There are two phases of intrathecal pump placement; trial and permanent placement. All patients must undergo a psychological assessment by a neuropsychologist prior to scheduling either the trial or the permanent pump.
STEP 1
Intrathecal Pump Trial The physician inserts a catheter into the intrathecal space of the spine, tunnelled under the skin and connected to an external pump.
STEP 2
The patient will be admitted for 23 hour observation to determine if the pump will help the patient. If the pain decreases during the observation period with the trial pump in place, the catheter will be removed and the physician may proceed with a permanent placement of the pump.
STEP 3
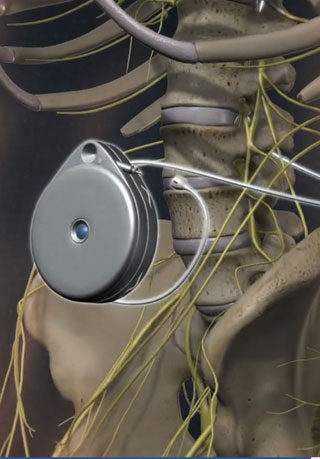
Permanent Intrathecal Pump – The permanent pump is usually placed under general anesthesia. The permanent pump is placed in the abdominal subcutaneous pocket while the permanent catheter is inserted into the intrathecal space of the spine, tunneled under the skin and connected to the pump. Staples or stitches are used to close the incision. The pump is filled with medication (usually morphine, hydromorphone, baclofen, bupivicaine, clonidine or a combination of these)
STEP 4
The pump is then programmed using an electronic programmer. The programmable pump allows clinicians to adjust doses, using the electronic programmer, non-invasively which minimizes patient discomfort. In addition, the pump can be programmed to deliver different doses at various times of the day, meeting patients’ changing needs.
STEP 5
The patient will be admitted overnight after placing the permanent pump to assure there are no adverse reactions. The patient should follow up within 5 days from the procedure to allow the physician to check the wound. The staples/stitches are removed in approximately 2 weeks.
INDICATIONS
More conservative therapies have failed, further surgical intervention is not indicated or possible, no serious untreated drug habituation exists, psychological evaluation and clearance for implantation has been obtained, no contraindications to implantation exist
POSSIBLE SIDE EFFECTS
The possible risks include: infection, granuloma or paralysis. Please discuss these with your physician.